Autoimmune dermatomyositis manifesting after receiving COVID-19 vaccine
Prashant Kaushik M.D., Chief, Division of Rheumatology, Northeastern Health System, Associate Program Director, Internal Medicine Residency Program, TMG/OMECO at the NHS, Associate Professor, Department of Internal Medicine, Oklahoma State University Center for Health Sciences, Tahlequah, OK
Muneeza Afif, M.D., Program Director, Internal Medicine Residency Program, TMG/OMECO at the NHS, Tahlequah, OK
Creticus P. Marak, M.D., Chairman of Department of Medicine and Pulmonologist, NHS, Tahlequah, OK
Jon D. Wilson, M.D., Director of Neuropathology, Arkana Laboratories., 10810 Executive Center Drive, Suite 100, Little Rock, AR 72211
Rabbiya Nisar, M.B., B.S., Auditioning prospective resident, NHS/TMG Internal Medicine Residency Program, Tahlequah, OK
Roger Montgomery, M.D., Executive Medical Director, Cherokee Nation Health Services; William W. Hastings Hospital, Tahlequah, OK
Funding: None. The authors do not have any conflict of interest. All authors had access to the data and a role in the formation of this manuscript.
Key words: COVID-19 vaccination, Oklahoma, Dermatomyositis
Introduction
Ours is a small rural Internal Medicine residency program at the Northeastern Health System, a unique health care facility in Tahlequah, Oklahoma, capital of the Cherokee Nation where the first Division of Rheumatology was started in January 2021. Worthy of noting is that COVID-19 vaccine has been approved by the FDA for the potentially serious COVID-19 spectrum.
Case: A 69-year old lady presented with worsening weakness of the proximal musculature of the pectoral and pelvic girdles along with a facial heliotrope rash. The symptoms started about one month after finishing Pfizer COVID-19 mRNA BNT162b2 vaccine. There was no local cellulitis at the injection-site or the arm. The muscle weakness worsened significantly along with the development of a macular, violaceous erythema overlying the right elbow, knuckles (Gottron’s sign), and upper chest (V sign). The patient was previously very active physically, but was now requiring extensive help with getting up and ambulation. Of note, the patient was free of any family history of any systemic inflammatory immune-mediated rheumatologic diseases (SIIRD).
Initially, the laboratory work-up revealed a normal white blood cell count, hemoglobin and platelet count and a normal serum creatinine, as well as C-reactive protein levels. However, there was a marked elevated serum creatine kinase (CK) level of 1340 (upper limit of normal 135 U/L). Serum lactate dehydrogenase and aldolase levels were normal. Anti-nuclear antibodies were positive in 1:160 titer, speckled pattern. Speciation was negative for SS-A, SS-B, RNP, Smith, Smith/RNP, chromatin, Scl 70, Jo-1, centromere and double stranded DNA antibodies. Complement C3 and C4 levels were normal. Myeloperoxidase and proteinase 3, c-ANCA, and p-ANCA antibodies were all negative. Hepatitis B surface antigen, surface antibody and core antibody were all negative. Hepatitis C antibody was nonreactive in the patient.
Given the association of dermatomyositis with an underlying occult visceral malignancy, a very dedicated work-up including malignant biomarkers carcinoembryonic antigen, CA 19–9, and CA- 125, a computerized tomography of chest, abdomen and pelvis, a serum and urine protein electrophoresis and a whole-body positron emission tomogram was normal/negative/unrevealing.
Electromyography and nerve conduction studies done on the right upper and lower extremities (intentionally sparing the left side for a possible muscle biopsy) were consistent with inflammatory myopathy. A left deltoid muscle biopsy was consistent with dermatomyositis (Fig. 1).
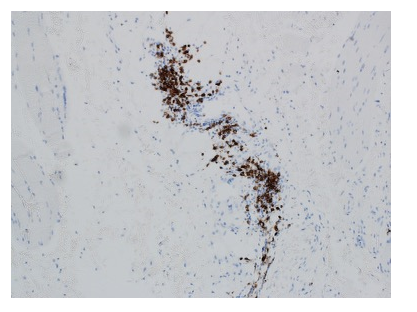
Immunofluorescence staining showed abundant CD20 cells in the inflamed areas (Fig. 2).
Figure 1. Patchy areas within the muscle biopsy showed the presence of an active myopathic process with pale staining necrotic muscle fibers and occasional basophilic regenerating muscle fibers (hematoxylin and eosin stained frozen section; 200x original magnification). In other areas of the biopsy patchy perifascicular atrophy was present
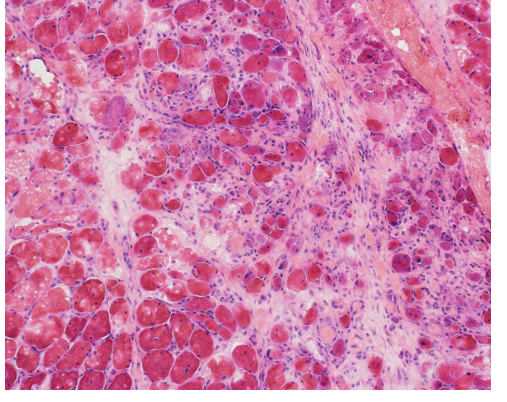
Figure 2. The chronic inflammatory infiltrate showed patchy mature appearing CD20-positive B-cells. The frequent CD3-positive T-cells were comprised of CD4 greater than CD8 positive lymphocytes [not shown].
The patient continued to develop worsening proximal muscle weakness despite the use of high-dose oral corticosteroids i.e. prednisone 1 mg/kg body weight and oral methotrexate 15 mg once a week along with folic acid supplementation. Surveillance liver chemistry showed a greater than three-fold elevation in the serum transaminases that persisted despite lowering the dose of methotrexate, necessitating its discontinuation. Physical therapy for muscle reconditioning was continued.
In view of the worsening clinical symptomatology, an inadequate response to corticosteroids, transaminemia with methotrexate, and the abundance of CD20 cells on muscle biopsy, a shared informed decision was made to initiate rituximab infusions, with a good response clinically.
Discussion: The mRNA COVID-19 vaccine is a lipid nanoparticle-encapsulated, nucleoside modified mRNA vaccine that encodes the pre-fusion spike glycoprotein of the SARS-Cov-2 virus/ COVID-19. Immediate as well as delayed reactions at or extending from the injection site have been noticed. Revelation of systemic autoimmunity following COVID-19 vaccination is just becoming visible including the induction of systemic lupus erythematosus, rheumatoid arthritis, ANCA associated vasculitis and even myocarditis from all over the world.1,2,3
Conclusion: This patient had no history of a known SIIRD or allergies to medications or adverse reactions to vaccinations previously. Use of the Naranjo probability scale indicated a possible relationship between the clinical manifestations of dermatomyositis and COVID -19 vaccination in this patient.4 It is, to the best of our knowledge, the first observation of dermatomyositis with COVID-19 Pfizer mRNA vaccine from Oklahoma. This case suggests that in certain individuals, COVID-19 vaccination could trigger SIIRD including dermatomyositis. Larger studies are needed to elucidate this plausible association. In the meantime, mindfulness about this observation is recommended as worldwide COVID-19 vaccine efforts continue to help increase herd immunity.
References:
1. Witberg G, Barda N, Hoss S, et al. Myocarditis after Covid-19 Vaccination in a Large Health Care Organization. N Engl J Med. 2021 Dec 2; 385(23): 2132-2139. doi: 10.1056/NEJMoa2110737. Epub 2021 Oct 6. PMID: 34614329; PMCID: PMC8531986.
2. Hakroush S, Tampe B. Case report: ANCA-associated vasculitis presenting with rhabdomyolysis and pauci-immune crescentic glomerulonephritis after Pfizer-BioNTech COVID-19 mRNA vaccination. Front Immunol [Internet]. 2021; 12:762006. Available from: http://dx.doi.org/10.3389/fimmu.2021.762006.
3. Capassoni M, Ketabchi S, Cassisa A, Caramelli R, Molinu AA, Galluccio F, Guiducci S. AstraZeneca (AZD1222) COVID-19 vaccine-associated adverse drug event: A case report. J Med Virol. 2021 Oct;93(10):5718-5720. doi: 10.1002/jmv.27175. Epub 2021 Jul 8. PMID: 34214206; PMCID: PMC8426669.
4. Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, et al. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981; 30(2):239–45. 10.1038/clpt.1981.154.