Monitoring HIV patient populations at risk for metabolic syndrome secondary to concomitant HAART and second-generation antipsychotic therapy
Nasar Ansari, PharmD; Grand Strand Medical Center, Myrtle Beach, SC
Divya Akula, DO, Ascension St. John Medical Center, Tulsa, OK
Morgan Heitt, BS; Oklahoma State University Center for Health Sciences, Tulsa, OK
Christina Connel, PharmD, BCPS, AAHIVP; Oklahoma State University Medical Center, Tulsa, OK
John Bury, PharmD, BCPS, Oklahoma State University Medical Center, Tulsa, OK
Kelly Murray, PharmD, BCACP., Oklahoma State University Center for Health Sciences, Tulsa, OK
Corresponding Author: Morgan Heitt, OMS-II
Conflicts of Interest: No financial or other sources of support were provided during the development of this protocol. Kelly Murray reports a research grant from Eli Lilly and the National Institute of Allergy and Infectious Diseases. All other authors have nothing to report.
Key Words: Antipsychotics, AIDS, Antiretrovirals, Cytochrome P450, Drug interactions
ABSTRACT
Purpose
People living with HIV (PLWH) are at a higher risk for metabolic complications secondary to inflammation caused by the virus. Second-generation antipsychotics (SGAs) and protease inhibitors (PIs) cause metabolic side effects. These are compounded with concomitant therapy due to additive effects and pharmacological interactions, potentially increasing serum SGA concentrations. The objective of this study is to explore the prevalence of metabolic syndrome with concurrent SGA and PI therapy and evaluate current risk management practices.
Methods
A retrospective chart review of 200 randomly selected patients taking SGAs and highly active antiretroviral therapy (HAART) for at least six months was conducted. Monitoring for metabolic complications, the prevalence of metabolic syndrome, and dosage adjustments among patients taking SGAs, PIs, or HAART were compared.
Results
Fifty-eight patients met the inclusion criteria for this study. Twenty met metabolic syndrome criteria, but zero had corresponding International Statistical Classification of Diseases (ICD-10) codes listed. Of these 20 patients, 13 took a PI, but none were monitored per the American Diabetes Association (ADA) recommendations. Furthermore, 44 patients took a PI. Most patients took SGAs with the highest metabolic risk, including quetiapine (55.2%) and olanzapine (10.3%). Of patients taking SGAs with recommended dose adjustments, only 3 of 34 (8.8%) were dosed appropriately.
Conclusion
This study highlights the need to develop a system of identifying PLWH undergoing HAART who require close management of metabolic syndrome. The results of this investigation have served as an incentive for heightened observation, including attention to patients’ psychiatric, metabolic, and HIV care.
INTRODUCTION
Worldwide, 37.7 million people were living with human immunodeficiency virus (HIV) or acquired immunodeficiency syndrome (AIDS) in 2020, of which one million were in the United States.1 Nearly two million new diagnoses and one million deaths are reported yearly.2 According to a multicohort study that looked at causes of death in nearly 50,000 HIV/AIDS-infected individuals, the leading causes of death were AIDS-related (29%), non-AIDS defining cancer (15%), liver disease (13%), and cardiovascular disease (CVD) (11%).3 Globally, twenty-one million PLWH are taking highly active antiretroviral therapy (HAART).1 With therapeutic advancements, the life expectancy of PLWH has increased significantly over time. Thus, the focus is shifting from mortality to morbidity reduction.
Among growing areas of HIV research is the pathophysiology of metabolic diseases in this patient population. Recognition of the contributory processes is crucial given that CVD is a leading cause of mortality in PLWH. These concerns can be further attributed to the pathology of metabolic syndrome, a collective term of risk factors for CVD, including elevated triglycerides, elevated fasting plasma glucose (FPG), reduced high-density lipoproteins (HDL), large waist circumference, and elevated blood pressure. Patients must have a diagnosis or treatment of three or more risk factors to meet the criteria for metabolic syndrome.4 In the United States, the prevalence of metabolic syndrome among the general population is approximately 30%.5 Although prevalence data for PLWH and metabolic syndrome in the United States are limited, research has shown that the incidence of metabolic diseases is higher than those without HIV.5
Globally, the risk of CVD and heart failure in PLWH is 1.5-2-fold greater than in non-infected people.6 A meta-analysis that compared over 60,000 PLWH taking HAART to treatment-naive individuals found the prevalence of hypertension to be 35% and 13%, respectively.7 PLWH and diabetes mellitus (DM) have a 2.4-fold greater risk of coronary heart disease-related events.6 Additionally, lower cluster of differentiation 4 (CD4) cell counts (<200 cells/mm3) are associated with a higher prevalence of atrial fibrillation and myocardial infarction.6 Furthermore, obesity among PLWH continues to increase with HAART initiation, even with lower risk, more effective regimens.6
HIV imposes a widespread chronic state of inflammation that causes multi-organ damage and precipitates insulin resistance, CVD, obesity, and liver disease. Monocytes, a type of white blood cell that differentiates into macrophages and other inflammatory cells, are dysregulated by HIV. As a result, monocyte-mediated expression of tyrosine kinases, negative inhibitors of inflammation and HIV transcription, is decreased.8 Additionally, HIV and HAART reduce adipocyte size, increasing circulating fats which are then deposited in muscle and the liver.9 Over time, this can lead to complications such as obesity and fatty liver disease.
Among HAART, protease inhibitors (PIs) have the most significant risk of metabolic toxicities. In addition to the above processes, PIs inhibit glucose transporter type 4 (GLUT-4), an insulin-dependent transporter that carries glucose to adipose and muscle tissue.8,9 This causes a rise in serum glucose. Also, altered lipid metabolism produces free radicals causing widespread damage, including pancreatic beta cellular injury that decreases insulin production.8 The subsequent decrease in insulin, which is required for blood glucose reduction and GLUT-4 mediation, coupled with the direct blockade of GLUT-4 by PIs, eventually causes insulin resistance. This can ultimately lead to diabetes and other complications, including metabolic syndrome.
Until 2018, the Department of Health and Human Services (DHHS) recommended both PI and integrase strand transfer inhibitor-based (INSTI) HAART regimens as first-line therapy. While INSTIs are preferred for initiation of treatment, PIs such as darunavir can be considered for specific populations and remain a commonly used drug class for various reasons.10 PIs have a very high genetic barrier to viral resistance, are available in a single-pill regimen, and are well-tolerated. Conversely, their significant potential for drug interactions and risk of metabolic complications has made them a less favorable option.
The widespread use of PIs necessitates heightened caution when managing associated risks. PIs are potent cytochrome P450 3A4 (CYP3A4) inhibitors that can increase the blood level of substrates, including statins, antithrombotic medications, and neuropsychiatric medications. Medications whose metabolism is strongly inhibited by CYP3A4 inhibitors may have more than a five-fold increase in the area under the curve (AUC).11 This risk is especially alarming for interactions that result in drug toxicities. Several SGAs are CYP3A4 substrates, which can cause increased metabolic side effects when used with PIs. These interactions further increase the risk of metabolic toxicities with this antiretroviral class. Of these SGAs, aripiprazole and quetiapine have specific dose adjustment recommendations, as use with a CYP3A4 inhibitor can result in an increase of the AUC by 1.5-2-fold and 3.4-6.2-fold, respectively.12, 13 It is recommended that aripiprazole be initiated at half the usual dose, with quetiapine being prescribed at one-sixth of the conventional dose when given with a strong CYP3A4 inhibitor.12, 13
Many patients rely on antipsychotics for the management of mood and psychotic disorders. As a result, SGAs are generally preferred over older, first-generation antipsychotics (FGAs) as metabolic side effects are better tolerated and managed than the extrapyramidal symptoms (EPS) of FGAs. However, many people who require SGAs are at high risk for metabolic disease independent of antipsychotic therapy. For example, more than two-thirds of people living with schizophrenia, a population that commonly uses SGAs, ultimately die from coronary heart disease.14 This risk increases when these patients undergo treatment with SGAs which have shown clear trends in causing weight gain, dyslipidemia, or diabetes independent of HAART therapy.15 The ADA addressed this increased risk by providing guidelines for preventing and managing metabolic syndrome associated with SGAs, particularly those taking medications with increased risk, such as olanzapine, clozapine, quetiapine, and risperidone.15 The ADA recommendations include routine monitoring of weight, lipids, glucose, and blood pressure. In 2014, the HIV Medicine Association Primary Care Guidelines also provided similar suggestions for monitoring, including fasting blood glucose, fasting lipid panels, blood pressure, and bone densitometry.16
This study explored the prevalence of metabolic syndrome among PLWH taking HAART and SGAs. It examined the monitoring and management practices at the Oklahoma State University (OSU) Internal Medicine Specialty Services Clinic (IMSSC). This is a longitudinal residency clinic for OSU's internal medicine residency program. The clinic is funded through the Ryan White Grant Program and served approximately 1,300 PLWH across eastern Oklahoma when this study was conducted. As a residency teaching clinic, we anticipated that monitoring and management practices could be improved in co-morbid patients.
METHODS
The hospital's information technology personnel obtained a randomized selection of 200 PLWH from the electronic medical record (EMR). To be included, patients had to have taken HAART and an SGA for six months between August 31, 2015, and September 30, 2018. Patients who had a change in HAART were excluded. Data were incomplete for those with inaccessible baseline information documented prior to a change in EMR in 2015.
A chart review of each subject was completed to evaluate risk factors for metabolic syndrome, ICD-10 diagnosis of metabolic syndrome, monitoring per ADA recommendations, and medication adjustments. A data collection tool was developed to stratify patients per their metabolic risk factors and other parameters (Table 1).
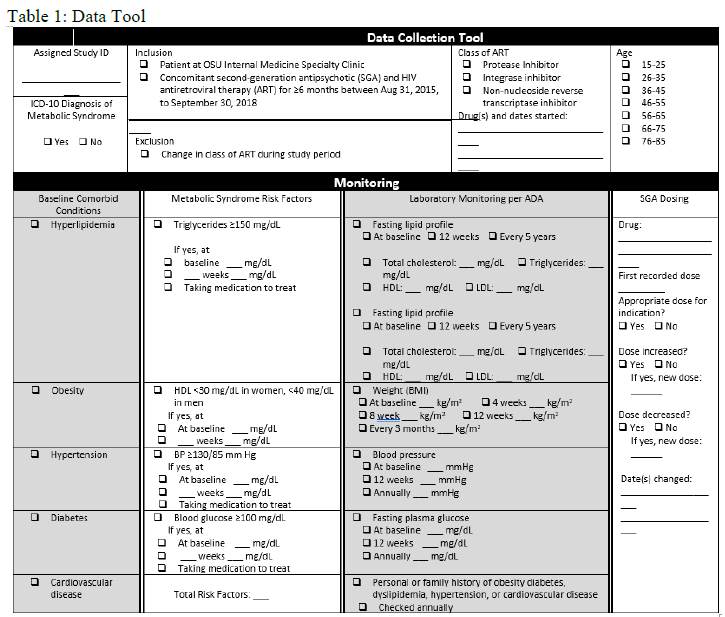
Prevalence of baseline HLD, obesity, hypertension, diabetes, and CVD was assessed through chart review or determined using the following definitions. Hyperlipidemia was defined using reference ranges built into the EMR. Normal ranges were cholesterol 100 - 199 mg/dL, triglyceride 0 - 149 mg/dL, HDL >39 mg/dL, very-low-density lipoprotein (VLDL) cholesterol (calculated) 5 - 40 mg/dL, and LDL (calculated) 0 - 99 mg/dL. Obesity was defined as body mass index (BMI) ≥30 by the Centers for Disease Control (CDC), and hypertension as blood pressure ≥130/85 mm Hg.4, 17 DM was recorded per provider diagnosis. CVD was assessed using World Health Organization (WHO) criteria, including coronary heart disease and peripheral vascular disease.18
Patients with metabolic syndrome have at least three of five risk factors for CVD: triglycerides ≥150 mg/dL, fasting blood glucose ≥100 mg/dL, HDL ≤40 mg/dL in men or ≤50 mg/dL in women, blood pressure ≥130/85, and large waist circumference.4 However, waist circumference is not routinely measured in clinical practice and was not assessed in this study. The data tool was also used to identify patients who had received at least six months of SGA therapy and HAART, and who met the criteria for metabolic syndrome, laboratory monitoring, and SGA dose adjustments. Statin dose appropriateness was later evaluated as well.
Each patient’s findings were entered into a Microsoft Excel spreadsheet to perform descriptive analysis. Patients who met the criteria for metabolic syndrome were further examined to note if a diagnosis was made. Additionally, investigators looked at how many patients were monitored per ADA guidelines for those taking SGAs. The primary objective was to identify metabolic syndrome among patients taking concurrent SGA and PI therapy. The secondary objectives were to evaluate monitoring practices and management of potentially contributory drug interactions among patients at risk for metabolic complications.
The study was approved by the OSU Center for Health Sciences Institutional Review Board. Microsoft Excel was used to organize data. Descriptive statistics were conducted to analyze study points.
RESULTS
Fifty-eight subjects met the inclusion criteria of concomitant SGA and HAART usage. Thirty-one of 58 patients were taking a PI-based regimen. Additionally, 13 patients were taking a combination regimen, all of which included a PI and a drug from another core class, such as an INSTI. The combined frequency of PI use was 44 of 58 patients (76%). Thirteen patients (22.4%) were taking an INSTI-based regimen, and one patient (1.7%) took a non-nucleoside reverse transcriptase inhibitor (NNRTI).
Within the study population, a majority of patients were taking quetiapine (46.6%), followed by nine (15.5%) taking aripiprazole, five (8.6%) taking both quetiapine and aripiprazole, six (10.3%) taking olanzapine, five (8.6%) taking risperidone, three (5.2%) taking ziprasidone, and one (1.7%) taking each of the following: lurasidone, iloperidone, paliperidone. Thirty-four patients took quetiapine and aripiprazole with a PI, and only three (8.8%) were appropriately dose adjusted. Additionally, one patient was taking a contraindicated regimen of lurasidone and a PI.
The prevalence of baseline comorbidities, including HLD, obesity, hypertension, DM, and CVD were assessed (Table 2).
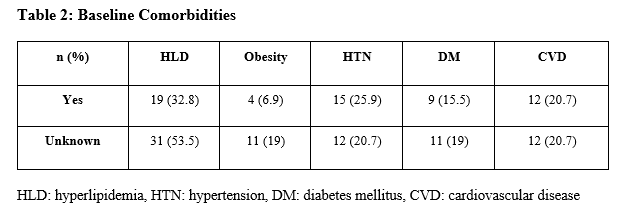
For most conditions, data were lacking for roughly 20% of patients. However, 53.5% of subjects did not have a baseline lipid panel, did not have a diagnosis of HLD, nor were they receiving treatment for HLD; the reason for this was unknown. Of those with accessible history, 32.8% had hyperlipidemia, 6.9% were obese, 25.9% had hypertension, 15.5% had diabetes, and 20.7% had cardiovascular disease at baseline. Twenty of 58 patients (35.4%) met the criteria for metabolic syndrome. Eight (13.8%) did not have an adequate monitoring history to make a determination. Of patients with metabolic syndrome, 13 of 20 (65%) were taking a PI, six (30%) were taking an INSTI, and one (5%) a NNRTI. None of the 20 patients identified who met diagnostic criteria had a documented diagnosis of or an ICD-10 code of metabolic syndrome. Of patients who had elevated triglycerides, reduced HDLs, or were treated with a lipid-lowering agent, 17 of 24 (71%) were taking a regimen that included a PI. Of those on a PI-based regimen, 64.7% took an appropriately dose-adjusted statin therapy.
While metabolic syndrome may not be routinely identified due to its nature as a cluster diagnosis, DM is more commonly monitored. However, among the nine patients in this study who had a diagnosis of DM at baseline, there was significant variability in which ADA monitoring recommendations for patients taking SGAs were followed (Table 3).
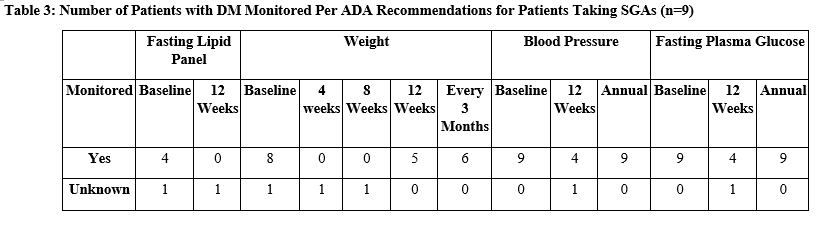
Baseline weight, blood pressure, and FPG were almost always recorded. A baseline fasting lipid panel was only recorded for four patients and unknown for one. Annual monitoring recommendations, including blood pressure and FPG, were recorded for all nine patients. The remaining parameters were not regularly monitored.
DISCUSSION
This study provided data highlighting the need for increased awareness in preventing and treating metabolic diseases among PLWH. The prevalence of psychiatric illness among this population is associated with an unhealthy lifestyle and increased stress, both likely contributors to metabolic syndrome.
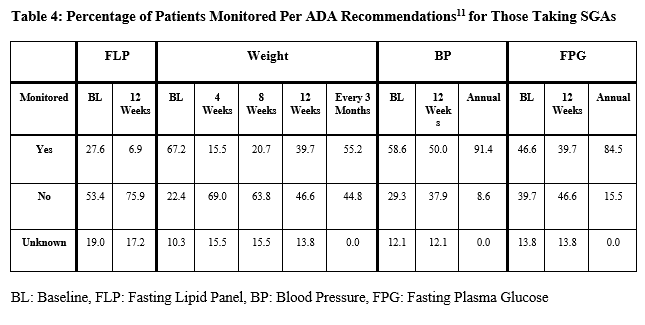
In this investigation, the SGAs that were most commonly prescribed were also those that carry the most significant risk of metabolic toxicities. Quetiapine and olanzapine have the highest risk for metabolic side effects, followed by risperidone, which carries a moderate risk, and finally, aripiprazole which holds the lowest risk.19 Of those treated with quetiapine, a majority were taking a non-dose-adjusted 200-400 mg daily. However, these patients were not consistently monitored per ADA recommendations (Table 4).
Patients prescribed high-risk SGAs develop metabolic syndrome at an average of 12.4 weeks. Still, it is reversible, especially if the offending drug is discontinued or adjusted within two months of diagnosis.20 Such findings underscore the importance of routine monitoring for metabolic side effects in those taking SGAs.
In this study, 76% of patients were taking a PI along with SGA therapy. Only three out of 34 patients taking quetiapine or aripiprazole with a PI had an appropriate dose adjustment in our study population. One patient was also prescribed a contraindicated regimen of lurasidone with a PI. Lurasidone inhibits the metabolism of PIs through CYP3A4, increasing the likelihood of metabolic effects and EPS.21 Given this reality, our recommendation for heightened metabolic monitoring should also correlate with clinical judgement on if PIs and concomitant SGA therapy should be transitioned in patient with risk factors if a concern for metabolic syndrome develops.
The ADA monitoring guidelines for at-risk patients taking SGAs include frequent screening for elevated blood sugar, blood pressure, lipids, and weight. For example, weight is checked at baseline, 4, 8, and 12 weeks, followed by every three months (Table 4). Most HIV monitoring guidelines predate recent pathophysiological findings and are based on older therapies whose risk profiles differ from modern HAART. However, in the last decade, guidelines have evolved with the understanding of metabolic diseases associated with HIV. The HIV Medicine Association Primary Care Guidelines expanded on the 2003 Infectious Disease Society of America (IDSA) guidelines for lipid management among PLWH. They included several recommendations for monitoring various metabolic parameters.15, 22 Additionally, the DHHS has also provided similar recommendations for monitoring. However, these do not consider modifying factors such as additional medications that may significantly impact metabolic parameters and cardiovascular risk.23 In recent years, the American Heart Association (AHA) stated that the pathophysiology of atherosclerotic cardiovascular diseases (ASCVD) in PLWH differed from the general population and suggested that the ASCVD risk calculator may not be appropriate for this population. However, in 2019, the AHA published its first detailed recommendations for ASCVD risk assessment in PLWH.6 It proposed using this calculator in conjunction with other considerations, including the diagnosis of metabolic syndrome, to identify patients who should receive statins and other medications to monitor and manage their metabolic diseases.
A multifaceted approach is required to create a realistic plan for preventing and treating metabolic syndrome among PLWH taking both SGAs and PIs. The new AHA recommendation for baseline assessment, followed by annual reassessment, should be considered for patients who are considered high risk. It may be argued that patients taking PIs and higher-risk SGAs qualify for heightened monitoring, especially if they have any risk factors at baseline. In this study, none of the patients were adequately monitored per the ADA guidelines, and the majority of the sampled subjects were taking higher-risk SGAs at non-dose-adjusted levels.
Additionally, newer evidence suggests that INSTIs may also be linked to cardiometabolic complications.24 INSTIs are also likely to be prescribed more frequently as they are first-line HAART. Therefore, close monitoring of metabolic parameters is warranted. Additionally, two INSTI-based regimens contain cobicistat, a pharmacoenhancer whose action is mediated via CYP3A4 inhibition. Cobicistat can increase the drug levels of CYP3A4 substrates such as SGAs, as is seen with concomitant use with PIs.
According to a study published by the ADA, only one-third of patients on SGA therapy are appropriately monitored.25 This data was from multiple sites in the United States and reviewed over 100,000 subjects who started SGA therapy. In addition, an investigation conducted by the University of California San Diego HIV Neurobehavioral Research program found that of 2,229 PLWH, 258 were taking HAART combined with SGA therapy. While the study recognized that PIs can cause HLD, diabetes, and obesity, it notes that HIV, some non-PI HAART, and SGAs all independently contribute to metabolic disease. They also found that quetiapine was the most commonly prescribed SGA and noted the CYP-mediated interaction with many HAARTs. The researchers emphasized the need for side effect management through lifestyle modification in combination with pharmacotherapy. Cognitive behavioral therapy and dietician consultation were also recommended when possible. The study also highlights the need for a more robust, prospective, and longitudinal review.
Existing data and the findings of our study speak to a widespread issue that needs immediate attention, especially for the use of a common drug class in a nation where cardiovascular disease and diabetes are leading causes of death. An algorithm for the management of drug interactions would be a useful tool in such interdisciplinary clinical settings. The AHA guidelines that speak specifically to metabolic risk management among PLWH should be widely utilized.6 Furthermore, pharmacists can play a vital role in interprofessional education and implementation of preventative measures that may include required monitoring, pharmacotherapy for side effect management, and dose adjustments.
ADA recommendations for required monitoring frequency may be arduous for clinicians to follow. Most patients are unable to visit their clinic repeatedly and may lack insurance coverage for such extensive screening. For patients monitored every six to twelve months but are developing or have worsening metabolic disease, three-month metabolic laboratory monitoring should be considered for necessary management. If a patient meets the criteria for metabolic syndrome, adding a diagnosis code to cover and justify appropriate screening may help increase the ability to monitor more appropriately. This would allow for earlier diagnostic identification, leading to a more proactive approach among clinicians in helping curb the incidence of metabolic syndrome in PLWH. This patient-centered approach should include lipid panels, blood pressure measurements, BMI assessments, and FPG analyses at each subsequent visit after patients begin HAART. Additionally, lifestyle modification and education that includes smoking cessation, eating a healthy diet, and exercise should be emphasized. It is worth noting that among PLWH, 42% were found to be current, and 20% were former smokers.5 Finally, in patients with newly diagnosed HIV living with cardiovascular comorbidities, we further recommend aggressive lifestyle alterations via dietitian intervention and clinical consideration of more weight-neutral SGAs as the psychiatric medication of choice.
This study was limited by sample size, missing baseline data in a significant proportion of the sample, a lack of comparator groups, and the inability to assess patients from the introduction of risk-modifying drugs (i.e., PIs or SGAs). While the clinic serves thousands of patients each year, this study was restricted to a small number of subjects at one clinical site. Therefore, it may not be reflective of a larger population of PLWH. Additionally, data was collected by two clinicians through retrospective chart review, potentially resulting in missing information and variability in data that was subject to provider judgment. Furthermore, waist circumference was not able to be assessed via retrospective chart review. Finally, a change in the electronic medical record in 2015 limited access to certain data, resulting in missing values.
While descriptive statistics show trends in data, designing a study to evaluate differences with t-tests would be beneficial. Since this study’s primary objective was to identify metabolic syndrome among patients taking concurrent SGA and PI therapy, a two-sample t-test or chi-square analysis would be appropriate for further analysis of our data. However, we were only able to identify the occurrence of metabolic syndrome among patients taking concurrent SGA and PI therapy. To utilize this statistical method of analysis, we would need data for patients who were solely on HAART therapy and those who were solely on SGA therapy. Additionally, we would need to identify the incidence of metabolic syndrome among each patient group to note any statistical significance associated with the combination therapy of HAART and SGA in causing metabolic syndrome.
The strengths of this study included the low cost of carrying out a retrospective chart review, in addition to taking an interprofessional investigational approach and involving researchers who are closely involved in the care of this patient population. Having pharmacists and a physician discuss study approaches and complete patient assessments allowed for improvement of the process and data analysis. This study further serves to build upon our hypothesis that concomitant SGA and HAART usage can lead to an increased incidence of metabolic syndrome, enabling providers to tend to this population with a new level of awareness for metabolic syndrome criteria in their patients. Future research opportunities that build on this study include conducting an educational intervention on the risk of metabolic syndrome in patients on PIs and SGAs, appropriate prescriptive management recommendations, and comparing diagnosis and monitoring practices to these descriptive results.
CONCLUSION
HIV-infected individuals are at risk for metabolic diseases due to both a chronic inflammatory state imposed by the virus and the side effects of HAART. As the life expectancy of PLWH increases due to more effective therapy, more patients will require increased management of comorbidities. SGAs and other medications that have metabolic side effects increase the risk of such issues.
Caution must be exercised when high-risk medications are used together due to their combined toxicities and interactions that increase drug levels. This study shines a light on the need for careful monitoring of PLWH who are taking such drugs. With improved interprofessional education and the implementation of a prevention tool, providers will be better equipped to prevent and manage metabolic comorbidities among PLWH.
REFERENCES
1. UNAIDS. Fact Sheet – Latest statistics on the status of the AIDS epidemic 2020. Available at http://aidsinfo.unaids.org/. Accessed July 8, 2022.
2. Echecopar-Sabogal J, D’Angelo-Piaggio L, Chanamé-Baca DM, Ugarte-Gil C. Association between the use of protease inhibitors in highly active antiretroviral therapy and incidence of diabetes mellitus and/or metabolic syndrome in HIV-infected patients: A systematic review and meta-analysis [Internet]. International Journal of STD & AIDS. 2017; 29(5):443-452.
3. Smith, Colette J; Ryom, Lene; Weber, Rainer; Morlat, Philippe; Pradier, Christian; Reiss, Peter; Kowalska, Justyna D; de Wit, Stephane; Law, Matthew; El-Sadr, Wafaa; Kirk, Ole; FriisMoller, Nina; Monforte, Antonella d'Arminio; Phillips, Andrew N; Sabin, Caroline A; Lundgren, Jens D. Trends in underlying causes of death in people with HIV from 1999 to 2011 (D:A:D): a multicohort collaboration. Lancet. 2014; 384(9939):241-248.
4. Amihăesei, I, Chelaru L. Metabolic syndrome a widespread threatening condition; risk factors, diagnostic criteria, therapeutic options, prevention and controversies: an overview. Rev Med Chir Soc Med Nat Iasi. 2014;118(4):896-900.
5. Calza, L, Colangeli, V, Magistrelli, E, Rossi, N, Rosselli Del Turco, E, Bussini, L et al. Prevalence of metabolic syndrome in HIV‐infected patients naive to antiretroviral therapy or receiving a first‐line treatment. HIV Clin Trials.2017; 18: 110-7.
6. Feinstein MJ, Hsue PY, Benjamin LA, Bloomfield GS, Currier JS, Freiberg MS, Grinspoon SK, Levin J, Longenecker CT, Post WS; Characteristics, prevention, and management of cardiovascular disease in people living with HIV: a scientific statement from the American Heart Association. Circulation. 2019; e1-27.
7. Xu Y, Chen X, Wang K. Global prevalence of hypertension among people living with HIV: a systematic review and meta-analysis. J Am Soc Hyper-tens. 2017;11:530–540.
8. Paula AA, Falcão MC, Pacheco AG. Metabolic syndrome in HIV-infected individuals: underlying mechanisms and epidemiological aspects. AIDS Res Ther. 2013;10(1):32.
9. Flint O, Noor M, Hruz P, Hylemon PB, Yarasheski K, Kotler DP, et al. The role of protease inhibitors in the pathogenesis of HIV-associated lipodystrophy: cellular mechanisms and clinical implications. Toxicol Pathol 2009; 37:65–77.
10. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV.Department of Health and Human Services. Available at http://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf. Initiation of Antiretroviral Therapy. Accessed June 2, 2019.
11. U.S. Food and Drug Administration. 2017. Clinical drug interaction studies–study design, data analysis, and clinical implications. Guidance for industry.U.S. Food and Drug Administration.Available at https://www.fda.gov/downloads/drugs/guidances/ucm292362.pdf. Accessed June 10, 2019.
12. Aripiprazole. Lexi-Drugs. Lexicomp. Wolters Kluwer Health, Inc. Riverwoods, IL. Available at https://online.lexi.com/.Accessed May 10, 2019.
13. Quetiapine. Lexi-Drugs. Lexicomp. Wolters Kluwer Health, Inc. Riverwoods, IL. Available at https://online.lexi.com/.Accessed May 10, 2019.
14. Schumann SA, Ewigman B. Can metformin undo weight gain induced by antipsychotics? J Fam Pract. 2008;57(8):526–530.
15. Consensus Development Conference on Antipsychotic Drugs and Obesity and Diabetes. Diabetes Care. 2004; 27 (2) 596-601.
16. Aberg JA, Gallant JE, Ghanem KG, et al. Primary care guidelines for the management of persons infected with HIV: 2013 update by the HIV medicine association of the Infectious Diseases Society of America. Clin Infect Dis. 2014;58:e1–34.
17. CDC – Centers for Disease Control and Prevention. Defining Adult Overweight & Obesity. Available at https://www.cdc.gov/obesity/basics/adult-defining.html. Accessed June 2, 2022.
18. WHO – World Health Organization. Cardiovascular Disease (CVDs). Available at https://www.who.int/news-room/fact-sheets/detail/cardiovascular-diseases-(cvds). Accessed June 2, 2022.
19. Gibson M, Carek PJ, Sullivan B. Treatment of co-morbid mental illness in primary care: how to minimize weight gain, diabetes, and metabolic syndrome. Int J Psychiatry Med. 2011;41:127-42.
20. Chadda RK, Ramshankar P, Deb KS, Sood M. Metabolic syndrome in schizophrenia: Differences between antipsychotic-naïve and treated patients. J Pharmacology Pharmacother. 2013;4(3):176–186.
21. Lurasidone - CYP3A4 Inhibitors. Interactions. Lexicomp. Wolters Kluwer Health, Inc. Riverwoods, IL. Available at https://online.lexi.com/.Accessed May 10, 2019.
22. Dube MP, Stein JH, Aberg JA, et al. Guidelines for the evaluation and management of dyslipidemia in human immunodeficiency virus (HIV)-infected adults receiving antiretroviral therapy: recommendations of the HIV Medical Association of the Infectious Disease Society of America and the Adult AIDS Clinical Trials Group. Clin Infect Dis. 2003;37:613–627.
23. Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the Use of Antiretroviral Agents in Adults and Adolescents with HIV. Department of Health and Human Services. Available at http://aidsinfo.nih.gov/contentfiles/lvguidelines/AdultandAdolescentGL.pdf. Laboratory Testing. Accessed June 5, 2019.
24. Rebeiro PF, Jenkins CA, Bian A, et al. Risk of Incident Diabetes Mellitus, Weight Gain, and Their Relationships With Integrase Inhibitor-Based Initial Antiretroviral Therapy Among Persons With Human Immunodeficiency Virus in the United States and Canada. Clin Infect Dis. 2021;73(7):e2234-e2242. doi:10.1093/cid/ciaa1403.
25. Bloomgarden, ZT. American Diabetes Association Postgraduate Meetings—2011. Diabetes Care.2011; 34 (11) e164-e169.