Ramsay Hunt Syndrome and Concomitant Encephalitis: Diagnostic Challenge Mimicking Acute Stroke
Riley Smith (MS-3)
Kate Reasoner (MS-4)
Dylan York, DO (PGY-2)
Kathy Cook, DO
Abstract:
Ramsay Hunt Syndrome (RHS) is a rare complication of varicella-zoster virus (VZV) reactivation. Among the more serious extra-cutaneous complications of VZV is encephalitis, carrying a less favorable prognosis. Encephalitis has multiple etiologies from viral infections, bacterial infections, and autoimmune. Patients with encephalitis have symptoms of subtle deficits to complete unresponsiveness dictated by the pattern of neurological involvement of encephalopathy. The occurrence of Ramsay Hunt Syndrome (RHS) with concomitant encephalitis is obscure. This case report demonstrates the diagnostic challenge of a patient
condition resembling an acute ischemic stroke (AIS) or otherwise known as an acute cerebrovascular accident (CVA). In the setting of equivocal imaging modalities, obtaining a lumbar puncture can aid in the differentiation of these pathologies to yield an accurate diagnosis.
Introduction:
RHS (herpes zoster oticus) is characterized by the reactivation of latent varicella-zoster virus (VZV) in the geniculate ganglion, leading to disruption of cranial nerve VII. RHS routinely presents as a triad of acute peripheral facial palsy, otalgia, and vesicles in the auditory canal. Associated symptoms can also include altered taste perception, tongue lesions, hearing abnormalities, vestibular disturbances, and lacrimation 1. The syndrome impacts an estimated 5 out of 100,000 people annually 2. An infrequent neurological complication of herpes zoster, VZV encephalitis, occurs with an annual incidence of 5.3 out of 1,000,000 3. This report explores the abnormal presentation of a patient exhibiting the rare co-occurrence of both RHS and VZV encephalitis, seeking to highlight the potential diagnostic challenges in distinguishing such a presentation from more common cerebrovascular diseases.
Case Presentation:
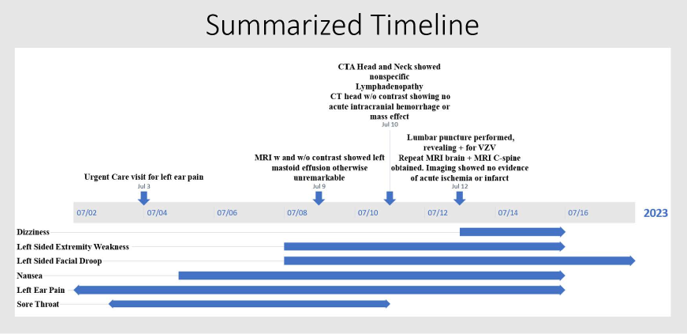
A 56-year-old female with a past medical history of an unspecified seizure disorder and hypertension presented to the emergency department (ED) with a complaint of left-sided weakness and facial droop. Six days prior, the patient had experienced the onset of a sore throat, described as “burning numbness,” followed by left ear pain, subjective fever, chills, nausea, and vomiting. This prompted a visit to an Urgent Care clinic, where she was given ciprofloxacin-dexamethasone ear drops and cefdinir for suspected bacterial otitis externa. Her ear pain did not improve and subsequently developed motor symptoms, prompting her to seek emergency medical care with concerns for stroke. She was hypertensive upon arrival to the ED, with a systolic blood pressure in the 170s. On physical examination, the patient had an NIH Stroke Scale score of 6, consisting of unilateral complete paralysis (upper/lower face), left upper extremity (UE) and left lower extremity (LE) motor drift, and decreased sensation to light touch and temperature on left UE/LE. Computed tomography (CT) of the head without contrast revealed no acute intracranial abnormality, hemorrhage, or mass effect. Following stroke protocol, CT angiography of the head and neck was then completed and revealed nonspecific mediastinal and cervical lymphadenopathy. While in the ED, the patient received cefdinir and ofloxacin for treatment of presumed otitis externa.
The patient was subsequently admitted to the general medical floor with the primary concern of AIS. While her unilateral complete facial palsy was suggestive of a lower motor neuron lesion, the presence of left upper and lower extremity prompted further evaluation for a lesion in the right middle cerebral artery or posterior circulation territories. At the recommendation of Neurology, the patient was initiated on medications for vascular risk reductions and magnetic resonance imaging (MRI) brain with and without contrast was obtained. MRI showed left mastoid effusion without bony erosion or periosteal enhancement. Although the exact location of presumed AIS was unable to be determined, it was noted that brainstem etiologies remain difficult to pinpoint on imaging modalities utilized.
Accompanying problems included concern for otitis externa with development into malignant otitis externa. The otoscopic examination was limited due an edematous external auditory canal (EAC) with a narrow lumen, precluding visualization of the tympanic membrane and proximal EAC. Otolaryngology was consulted, who noted a markedly inflamed left pinna, dried blood present diffusely around the ear canal, and the absence of otorrhea. Vesicles were not noted in the oral cavity or the ear. The patient was started on IV ciprofloxacin and
ciprofloxacin/dexamethasone external application drops. Despite treatment, her pain and bloody discharge continued, as shown in Figure 1 below. Throughout her hospital stay, the patient continued to experience throat discomfort. Speech-language pathology was consulted for odynophagia, and a modified barium swallow study was performed. Although the findings were unremarkable a soft diet was temporarily provided for patient comfort.
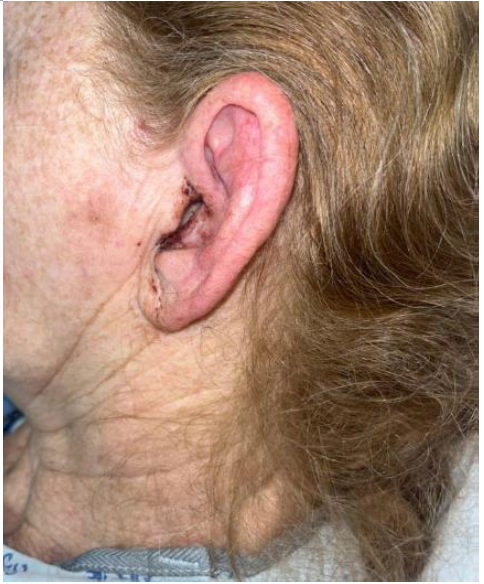
Figure 1. Left external ear (Note: photograph taken late in clinical course)
Repeat MRI of the brain with the addition of the cervical spine with and without contrast to evaluate for brainstem pathology was obtained, again revealing stable left mastoid effusion without acute findings. A second opinion from neurology expressed concern for spread to the meninges. The recommendation was made at this time to obtain a lumbar puncture and cerebrospinal fluid (CSF) analysis, including a comprehensive meningitis panel. Due to significant concerns about viral encephalitis, empiric treatment with intravenous acyclovir 10
mg/kg TID for a minimum duration of 14 days was initiated while awaiting the fluid study results show in Summary Table 1. Viral PCR studies were positive for VZV.
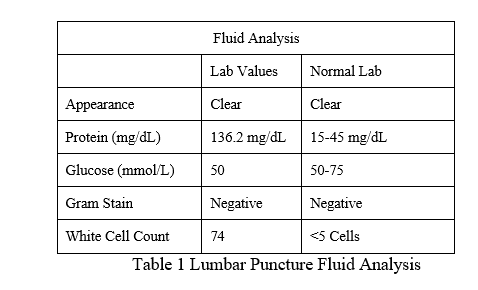
Following virologic confirmation, an additional physical examination of the oral cavity was conducted, revealing an erythematous vesicular rash limited to the left soft palate, as shown below in Figure 2. Symptoms proceeded to include worsening sensory aspects of the geniculate ganglion that lead to her being unable to tolerate oral intake.
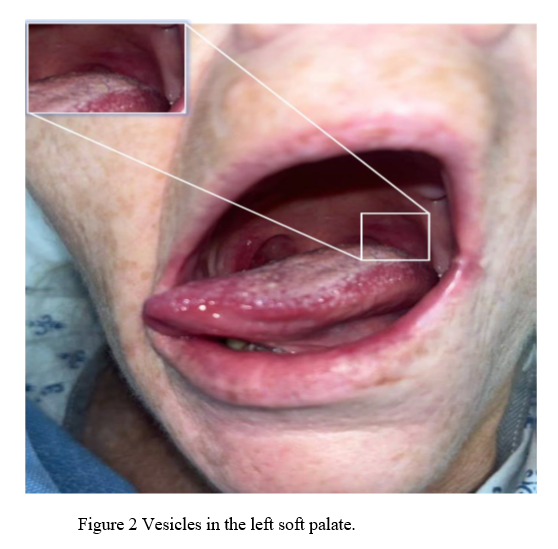
Clinical improvement occurred within 24 hours of starting empiric treatment. Over 48-72 hours, the patient’s minimal oral intake returned to baseline, and she no longer required assistance in ambulation. The patient was discharged home in stable condition with supplies for self-administration of intravenous acyclovir. Although there was no evidence of AIS on imaging, the patient was instructed to complete 21 days of full-dose aspirin and clopidogrel to mitigate the risk of cerebrovascular complications during the peri-encephalitic period.
Discussion:
The onset of RHS involves the reactivation of latent VZV in the geniculate ganglion, leading to viral spread along the facial nerve, resulting in acute peripheral facial palsy. The inflammation due to VZV infection in the geniculate ganglion and surrounding structures produces the characteristic RHS clinical features. It is notable that early in the course of RHS, the condition may be clinically indistinguishable from Bell’s palsy, as the unilateral otalgia and ipsilateral facial palsy may precede the appearance of herpetic vesicles by two to three days4. Though Bell’s palsy is strictly defined as an idiopathic facial nerve palsy, it has been associated with herpes simplex virus reactivation in most cases5. Yet, despite their shared pathophysiologic qualities, Bell’s palsy and RHS differ in terms of prognosis. RHS may induce an inflammatory response in the facial nerve more severe than Bell's palsy, posing a higher risk of late neural denervation and a lower probability of full recovery6.
The complexities of diagnosis are further magnified by the phenomenon of zoster sine herpete (ZSH), where up to 30% of Ramsay Hunt cases may present without the hallmark rash7. While this patient was ultimately discovered to have an erythematous vesicular eruption on the left soft palate, the patient's otic eruption presenting as a blood discharge without vesicles prompted suspicion for tympanic membrane trauma or bacterial otitis media/externa. Likewise, the patient’s discrete palatal vesicles were not discovered until after the herpes zoster diagnosis was recognized by the detection of VZV DNA in the CSF. In the context of such concerning
symptoms as complete hemiparesis and concern for AIS, her subtle and variant cutaneous eruptions were overlooked, thus paralleling the challenges typical of ZSH.
Despite the potential absence of cutaneous signs of VZV reactivation, clinicians should still consider ZSH, particularly when evaluating atypical cases of acute peripheral facial palsy. For such cases, virologic testing can be especially helpful. Polymerase chain reaction (PCR) detection of VZV DNA is the most useful test, rapidly providing high sensitivity and specificity. Serologic assay of anti-VZV IgG/IgM immunoglobulins have been utilized, however, their effectiveness varies based on the stage in the clinical course. Additionally, oropharyngeal swab specimens may provide a convenient and sensitive means of testing. In a recent study
comparing saliva and plasma specimens, PCR assay showed significantly higher sensitivity (88%-100%) in the saliva of herpes zoster patients than in plasma (78%-100%)8.
The diagnosis of VZV encephalitis is primarily accomplished through CSF analysis with PCR detection of VZV DNA. However, a potential hurdle arises when patients exhibit focal neurologic deficits but lack obvious signs of encephalitis or clear indicators of infections with a risk of dissemination to the CNS. In such cases, patients may be presumed to have had an acute stroke, potentially delaying a lumbar puncture while prioritizing neurologic imaging studies to evaluate for AIS. Although CT and MRI may reveal VZV encephalitis, their sensitivities have been estimated to be as low as 30% and 66%, respectively9.
Differential Diagnosis
For the majority of this patient’s hospital stay, the primary assessment was considered to be AIS. Given the presence of hemiparesis in addition to the patient’s facial palsy, it was initially considered unlikely a Bell’s palsy-like pathology could explain the motor weakness other than in the distribution of cranial nerves, leading to the consideration of AIS. While the serious nature of AIS warrants consideration of such an explanation for patients’ neurologic deficits, especially if the patient presents within the window for thrombolysis and/or mechanical thrombectomy, an important principal in the decision to employ these therapies, however, is ruling out stroke-mimics by evaluating the distribution of neurologic deficits for localization to a reasonably likely cerebrovascular etiology. In the case of our patient, retrospective analysis of her deficits reveals our initial theory of a Diffusion Weighted Imaging (DWI)-negative AIS occurring in the poorly visualized posterior circulation as untenable. Strokes which produce false negative DWI do not appear on the DWI form of MRI imaging, but do so with characteristic scenarios. These associations are typically posterior circulation strokes, small volume infarcts, performing MRI within 6 hours of stroke onset, and non-disabling strokes (NIHSS <4)10. We gravitated toward an explanation of a brainstem stroke to explain the combination of facial palsy with hemiparesis, as well as because of the known association of DWI-negative imaging with infarcts in this region. However, of the brain stem stroke syndromes which cause facial palsy with hemiparesis, these occur in the pons with contralateral hemiparesis and ipsilateral facial palsy. The laterality of the patient’s findings undermines the feasibility of a DWI-negative stroke explaining her deficits.
Another potential stroke lesion to consider is a lacunar infarct in the posterior limb of the internal capsule. This choice is supported by the notion that a lacunar syndrome characterized by pure motor stroke might provide an explanation for the patient's hemiparesis. To illustrate, if our
investigation is primarily aimed at identifying a stroke syndrome to account for the hemiparesis while excluding lower motor neuron facial palsy, we can take the example of being informed about RHS and suspecting that a VZV vasculopathy could have triggered an AIS
resulting in hemiparesis. In this context, it's plausible to consider that an AIS affecting the posterior limb of the internal capsule could lead to a pure motor stroke, which in turn would cause hemiparesis. However, this mechanism is also improbable, as a VZV vasculopathy originating from RHS of the left geniculate ganglion is anatomically limited in its ability to affect the cerebrovasculature of the left hemisphere. Therefore, while it's conceivable that RHS may lead to VZV vasculopathy, which, in turn, could cause a left internal capsule AIS, it
would necessarily result in right-sided hemiparesis.
While DWI-negative ischemic strokes are not uncommon, accounting for 6.8% of ischemic strokes according to a meta-analysis of 3,236 cases11, the misidentification of stroke mimics as true vascular events is even more prevalent. The frequency of stroke mimics varies considerably depending on the clinical setting and the expertise of the evaluating healthcare provider. For instance, a study involving 8,187 patients who were referred to an acute stroke service found that 30% were actually experiencing stroke mimics12. Given the significant discrepancy in incidence rates between diffusion-negative strokes and stroke mimics—which constitute nearly
one-third of all suspected stroke cases—there is a pressing need to rigorously investigate the diverse range of conditions that can mimic a patient’s stroke symptoms.
In such scenarios where imaging proves equivocal, it may be prudent to consider CSF PCR analysis, regardless of the lack of classical signs of an encephalitic infection, such as fever, rhythmic muscle contractions, photophobia, and altered mental status. The high sensitivity and specificity of CSF PCR assay, exceeding 95%, accentuate its utility in diagnosing VZV encephalitis.13
Further, the importance of early diagnosis is highlighted by the differential outcomes for those patients initiated on treatment before and after 72 hours following symptom onset. In a study comparing treatment outcomes in RHS acute facial palsy (House-Brackmann scores ≥ 3), it was found that 75% of patients saw full resolution of facial palsy if acyclovir and prednisone were started within 3 days of symptom onset, whereas only 30% saw full resolution if treatment were started one week following onset. Without treatment, only 20% are expected to have any improvement2.
Conclusion:
This case report reveals the diagnostic challenges seen in atypical presentations of Ramsay Hunt Syndrome. The convergence of RHS symptoms with stroke-like manifestations of VZV encephalitis can pose significant challenges, often blurring the line between more prevalent conditions like cerebrovascular accidents. The phenomenon of zoster sine herpete acts as a reminder that the absence of the hallmark vesicular rash does not eliminate the potential for RHS. When clinical manifestations are incongruent and traditional imaging techniques offer no clear answers, the diagnostic lens should widen before attributing the clinical picture to
DWI-negative stroke. Given the proven linkage between swift therapeutic intervention and favorable outcomes in RHS, coupled with the risk of severe neurological consequences, clinicians should prioritize the early incorporation of RHS into their differential diagnosis. Knowledge of the spectrum of RHS manifestations not only empowers clinicians to intervene early and effectively but also emphasizes the importance of thorough physical examination, complemented by tools like lumbar puncture and PCR, in making an accurate diagnosis.
References
1. Crouch AE, Hohman MH, Moody MP, Andaloro C. Ramsay Hunt Syndrome. StatPearls Publishing; 2023. Accessed July 29, 2023. https://www.ncbi.nlm.nih.gov/books/NBK557409/
2. Murakami S, Hato N, Horiuchi J, Honda N, Gyo K, Yanagihara N. Treatment of Ramsay Hunt syndrome with acyclovir-prednisone: significance of early diagnosis and treatment. Ann Neurol. 1997;41(3):353-357. doi:10.1002/ana.410410310
3. Herlin LK, Hansen KS, Bodilsen J, et al. Varicella Zoster Virus Encephalitis in Denmark From 2015 to 2019-A Nationwide Prospective Cohort Study. Clin Infect Dis. 2021;72(7):1192-1199. doi:10.1093/cid/ciaa185
4. Van Le M. Image diagnosis: Ramsay Hunt syndrome. Perm J. 2012;16(4):51-52. doi:10.7812/TPP/12-035
5. Holland NJ, Bernstein JM. Bell’s palsy. BMJ Clin Evid. 2014;2014.
https://www.ncbi.nlm.nih.gov/pubmed/24717284
6. Sweeney CJ, Gilden DH. Ramsay Hunt syndrome. J Neurol Neurosurg Psychiatry. 2001;71(2):149-154. doi:10.1136/jnnp.71.2.149
7. Lee HY, Kim MG, Park DC, Park MS, Byun JY, Yeo SG. Zoster sine herpete causing facial palsy. Am J Otolaryngol. 2012;33(5):565-571. doi:10.1016/j.amjoto.2012.02.001
8. Park SY, Kim JY, Kim JA, et al. Diagnostic Usefulness of Varicella-Zoster Virus Real-Time Polymerase Chain Reaction Analysis of DNA in Saliva and Plasma Specimens From Patients With Herpes Zoster. J Infect Dis. 2017;217(1):51-57. doi:10.1093/infdis/jix508
9. Mirouse A, Sonneville R, Razazi K, et al. Neurologic outcome of VZV encephalitis one year after ICU admission: a multicenter cohort study. Ann Intensive Care. 2022;12(1):32. doi:10.1186/s13613-022-01002-y
10. Nagaraja N. Diffusion weighted imaging in acute ischemic stroke: A review of its interpretation pitfalls and advanced diffusion imaging application. J Neurol Sci. 2021;425:117435. doi:10.1016/j.jns.2021.117435
11. Edlow BL, Hurwitz S, Edlow JA. Diagnosis of DWI-negative acute ischemic stroke: A meta-analysis. Neurology. 2017;89(3):256-262. doi:10.1212/WNL.0000000000004120
12. Merino JG, Luby M, Benson RT, et al. Predictors of acute stroke mimics in 8187 patients referred to a stroke service. J Stroke Cerebrovasc Dis. 2013;22(8):e397-e403.
doi:10.1016/j.jstrokecerebrovasdis.2013.04.018
13. DeBiasi RL, Tyler KL. Polymerase chain reaction in the diagnosis and management of central nervous system infections. Arch Neurol. 1999;56(10):1215-1219. doi:10.1001/archneur.56.10.1215