Shelby Brown, DO, Oklahoma State University Medical Center, Tulsa, OK
Tschantre Dorsett, MD., W.W. Hastings Hospital, Tahlequah, OK
Corresponding Author: Alexander J. Eddy; alexander.eddy@okstate.edu; 19500 E. Ross St., Tahlequah, OK 74464
Statement of Funding: The authors did not receive funding for this case study.
No conflicts of interest or disclosures
Abstract
Ovarian teratomas are germ cell tumors that can be benign (mature) or malignant (immature). Individuals usually present with lower abdominal pain. Teratomas can grow larger than 30 cm and contain tissues derived from endoderm, mesoderm, and ectoderm. Here, we describe a 45-year-old nulligravida female with bilateral mature ovarian teratomas, the larger of which exceeded 11 cm. The patient’s enlarged, fibroid-filled uterus and ovaries were carefully extracted in the operating room and examined. Fragmentation of each teratoma yielded copious amounts of hair, teeth, and sebaceous material. Neither histology nor tumor markers indicated malignancy. We do not know whether the patient’s experience of substance use contributed to her condition or increased her risk for future malignant transformation.
Introduction
Mature and immature ovarian teratomas may be composed of derivatives of ectoderm, mesoderm, or endoderm.1 Immature teratomas are malignant by nature, whereas mature teratomas are benign. However, mature teratomas can undergo malignant transformation.2 Lower abdominal pain is the most commonly reported presentation of ovarian teratomas, and the mean age at which patients undergo surgical removal is 32.7 years. Ovarian torsion is a semi-frequent (approximately 5% of cases) complication that can occur with both teratomas.3 Ovarian teratomas present bilaterally in approximately 10-15% of cases.3,4 Immature teratomas are larger in diameter (15.3 cm vs. 7.5 cm, p=0.0004), on average.3 However, mature teratomas can reach massive sizes. Devoize et al documented a 32 cm, 10 kg mature teratoma with over 300 teeth, hair follicles, adipose tissue, glial tissue, and perinerval nervous tissue.5 Recurrence after surgery is rare but is most common in cases of incomplete surgical resection and immature teratomas.6
Case Report
A 45-year-old nulligravida woman with a BMI of 26 kg/m2 presented to the clinic for evaluation of uterine fibroids. She endorsed a multiyear history of abdominal pain and pressure along with dyspareunia. Additionally, she noted a 4-month history of abnormal, heavier periods with 7-8 days of bleeding compared to a previous average of 3-4 days. She denied hematuria, hematochezia, incontinence, headache, or vision changes. Social history was notable for a 30 pack-year smoking history, binge drinking, and methamphetamine use. She noted a history of ovarian cancer in her mother, leading to her mother’s demise at 71.
Transvaginal ultrasound showed an enlarged heterogeneous uterus (13.2x7.9x8.6 cm) secondary to multiple fibroids with a thickened endometrium (2.66 cm). Additionally, the left ovary was enlarged with what appeared to be a large left ovarian dermoid cyst (9.7x9.4x6.5 cm). The right ovary could not be visualized via transvaginal ultrasound, but transabdominal ultrasound showed it was also enlarged (11.5x7.8x11.3 cm) with what appeared to be another dermoid cyst. Tumor markers (with normal range in parentheses) were as follows: LDH 178 U/L (120-246 U/L), AFP 1.1 ng/mL (<6.1 ng/mL), CA125 22 units/mL (<35 units/mL), Inhibin A 28 pg/mL (<98 pg/mL premenopausal), Inhibin B <10 pg/mL (<153 pg/mL premenopausal), Beta hCG <2.4 mIU/mL (≤25 mIU/mL). Thus, all measured tumor markers were negative.
The patient was admitted for a total laparoscopic hysterectomy with bilateral salpingo-oophorectomy and cystoscopy. During surgery, the uterus was found to be too large to be evacuated through the vagina and had to first be fragmented. The left and right ovaries were also very large (Figure 1). Both ovaries were removed and placed on a tray for further examination. Fragmenting each ovary with its accompanying dermoid cyst revealed copious amounts of hair with little pigmentation, sebaceous fluid, keratin, and teeth (Figure 2). Approximately one dozen teeth were found, predominantly resembling molar teeth.
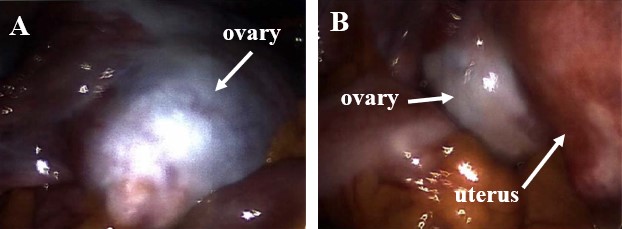
Figure 1. Enlarged ovaries (A and B), secondary to tumor growth, adjacent to an enlarged uterus (B), secondary to leiomyomata uteri.
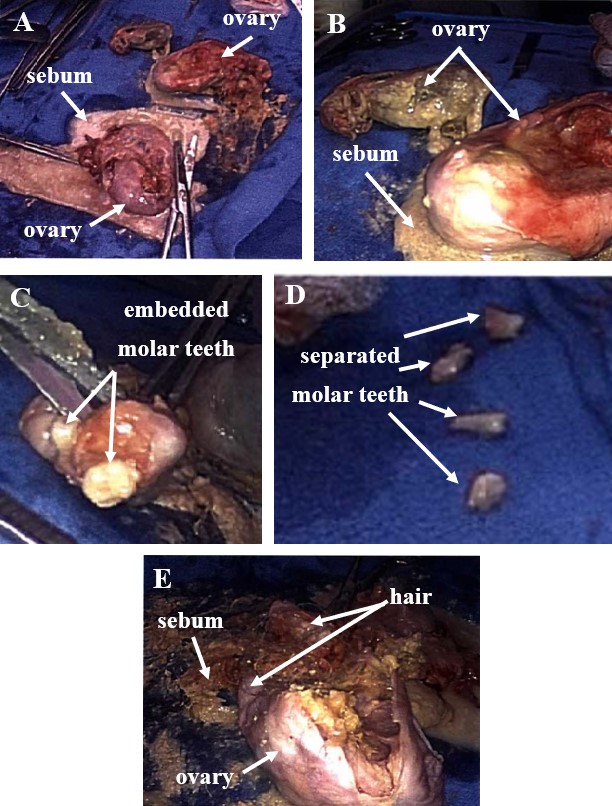
Figure 2. Both ovaries are shown placed on tray table with sebum spilling out (A and B). Teeth resembling molars are shown embedded in a tumor fragment (C). Some teeth have been manually separated (D). Enlarged view of teratoma showing hair and sebaceous material (E).
The patient recovered well and was discharged the next day. She had no complications on two-week post-op follow up and endorsed that most of her abdominal pain had resolved except for some soreness from her surgical wounds.
Discussion
The type of ovarian cancer the patient’s mother succumbed to was unknown. A large portion of ovarian neoplasms, as much as 25%, are germ cell tumors. However, they account for only about 5% of ovarian cancers.2 The proportion of mature teratomas that undergo malignant transformation is approximately 0.17-2%, and the most common resultant cancer is squamous cell carcinoma.2 Thus, it is unlikely that the patient’s mother’s cancer occurred from the transformation of a mature teratoma. Furthermore, the occurrence of familial ovarian teratomas is thought to be very rare and is not frequently referenced in the literature.7
Ovarian teratomas most commonly occur in women less than 45 years old.1 No clear relation in a case-controlled study of 77 women was found between benign ovarian teratoma and: parity, abortions, age at first pregnancy, age at menarche, menstrual cycle pattern, menopausal status, oral contraceptive use, and marital status. However, there was a statistically significant positive correlation between a higher level of education and the occurrence of benign ovarian teratoma.8Thus, the extent to which the patient’s lifestyle and external factors may have affected her risk is unknown. Regarding her risk of malignant transformation, it is unknown if the patient’s social history involving tobacco, methamphetamine, and excess alcohol increased her risk. Some suggest that long-term exposure of the ovaries to carcinogenic substances could cause malignant changes.2,9 Additionally, larger size is noted to be a risk factor for malignant transformation.10 Given the size of the tumor and the patient’s family history of ovarian cancer of a potentially different origin, a shared decision was made to carry on with a bilateral salpingo-oophorectomy along with the hysterectomy.
References
1. Saba L, Guerriero S, Sulcis R, Virgilio B, Melis G, Mallarini G. Mature and immature ovarian teratomas: CT, US and MR imaging characteristics. European Journal of Radiology. 2009;72(3):454-463. https://doi.org/10.1016/j.ejrad.2008.07.044
2. Hackethal A, Brueggmann D, Bohlmann MK, Franke FE, Tinneberg H, Münstedt K. Squamous-cell carcinoma in mature cystic teratoma of the ovary: systematic review and analysis of published data. The Lancet Oncology. 2008;9(12):1173-1180. https://doi.org/10.1016/S1470-2045(08)70306-1
3. Kim MJ, Kim NY, Lee D, Yoon B, Choi D. Clinical characteristics of ovarian teratoma: age-focused retrospective analysis of 580 cases. American Journal of Obstetrics and Gynecology. 2011;205(1):32.e1-32.e4. doi:10.1016/j.ajog.2011.
4. Chang, CF, Lin, CK. A case of recurrent, bilateral ovarian mature teratoma in a young woman. BMC Women's Health. 2014;14(57). https://doi.org/10.1186/1472-6874-14-57
5. Devoize L, Collangettes D, Le Bouëdec G, Mishellany F, Orliaguet T, Dallel R, Baudet-Pommel M. Giant mature ovarian cystic teratoma including more than 300 teeth. Oral Surgery, Oral Medicine, Oral Pathology, Oral Radiology and Endodontology. 2008;105(3):e76-e79. doi:10.1016/j.tripleo.2007.10.001
6. Terenziani M, D'Angelo P, Inserra A, Boldrini R, Bisogno G, Babbo GL, Conte M, Dall' Igna P, De Pasquale MD, Indolfi P, Piva L, Riccipetitoni G, Siracusa F, Spreafico F, Tamaro P. Cecchetto G. Mature and immature teratoma: A report from the second Italian pediatric study. Pediatr Blood Cancer. 2015;62:1202-1208. https://doi.org/10.1002/pbc.25423
7. Braungart S, McCullagh M. Management of Familial Ovarian Teratoma: The Need for Guidance. European J Pediatr Surg Rep. 2016;4(1):31-33. doi:10.1055/s-0036-1593832.
8. Parazzini F, La Vecchia C, Negri E, Moroni S, Villa A. Risk factors for benign ovarian teratomas. Br J Cancer. 1995;71:644-646. https://doi.org/10.1038/bjc.1995.127
9. Rim S, Kim S, Choi H. Malignant transformation of ovarian mature cystic teratoma. International Journal of Gynecologic Cancer. 2006;16:140-144. http://dx.doi.org/10.1136/ijgc-00009577-200601000-00023
10. Dos Santos L, Mok E, Iasonos A, Park K, Soslow RA, Aghajanian C, Alektiar K, Barakat RR, Abu-Rustum NR. Squamous cell carcinoma arising in mature cystic teratoma of the ovary: A case series and review of the literature. Gynecologic Oncology. 2007;105(2):321-324. doi:10.1016/j.ygyno.2006.12.008